38 fda approved drug labels
FDALabel - U.S. Food and Drug Administration Labeling, Product and Ingredient Identifiers. Application Number for ANDA, BLA, or NDA: 3 to 6 digits (e.g., 077844, 125118, 020977) Unique Ingredient Identifier (UNII): To search for active ingredients, inactive ingredients or both, type in alphanumeric code (s) (e.g., J220T4J9Q2) Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for use...
Drug Labels | FDA Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and...

Fda approved drug labels
Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Drugs@FDA: FDA-Approved Drugs. Share; Tweet; Linkedin; Pin it; More sharing options. Linkedin; Pin it; Email; ... Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/18/2016: SUPPL-14: Manufacturing (CMC) Label is not available on this site. 04/22/2016: Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions... What Information Should Be on Drug Labels? - MedicineNet Certain information must be included on a prescription drug label. The FDA requires prescription labeling to be printed with: Pharmacy information The doctor's information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered
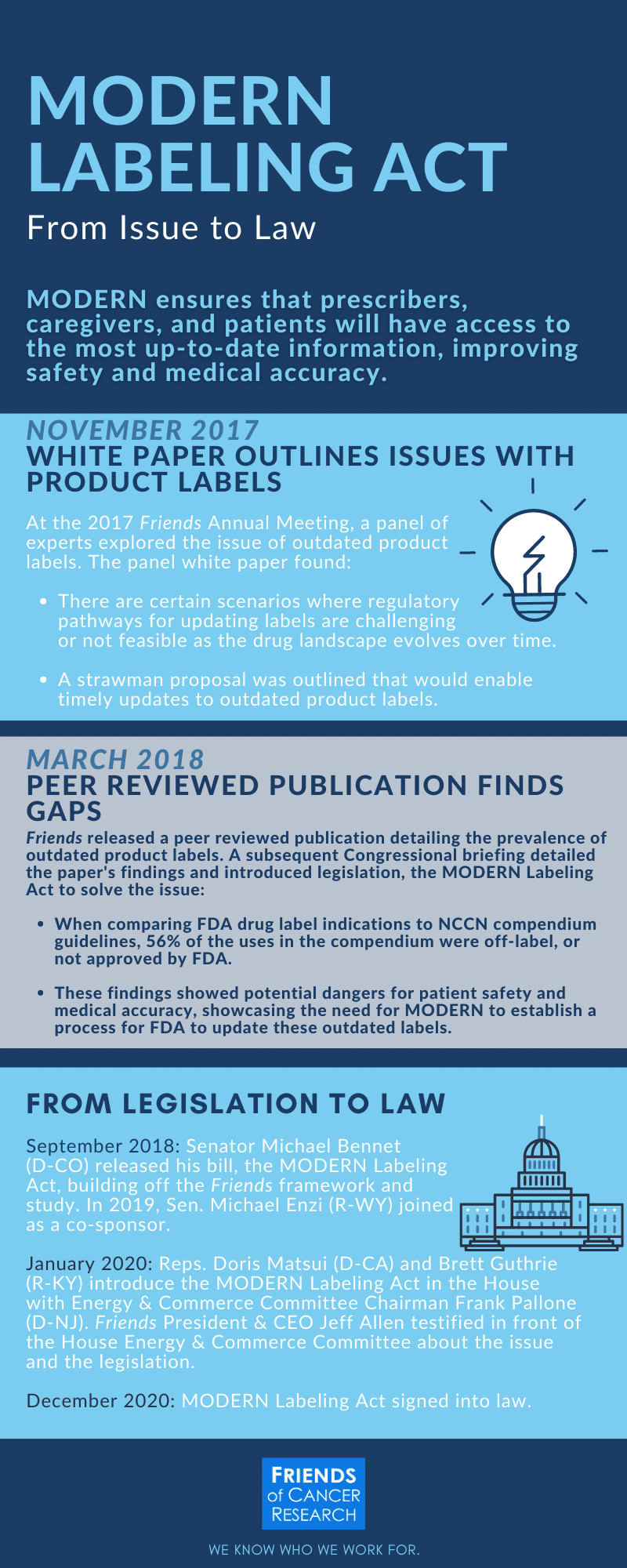
Fda approved drug labels. PDF TOPAMAX (topiramate) Label - Food and Drug Administration Suicidal behavior and ideation: antiepileptic drugs increase the risk of suicidal behavior or ideation (5.5) Cognitive/neuropsychiatric adverse reactions: use caution when operating machinery including cars; depression and mood problems may occur (5.6) Fetal Toxicity: use during pregnancy can cause cleft lip and/or palate and PDF HIGHLIGHTS OF PRESCRIBING INFORMATION The recommended dose is 2.5 mg ... Discontinue drug or discontinue nursing. (8.2) Severe Hepatic Impairment: Not recommended. (8.7, 12.2) See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 04/2021 . FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: (A) PREMATURE DISCONTINUATION OF ELIQUIS. INCREASES THE RISK OF THROMBOTIC EVENTS (B) SPINAL/EPIDURAL HEMATOMA Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web... FDA Approves Label Extension for Evrysdi for Infants with Spinal ... SOUTH PLAINFIELD, N.J., May 31, 2022 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that the U.S. Food and Drug Administration (FDA) has approved a label extension for Evrysdi ( risdiplam) to include infants under 2 months old with spinal muscular atrophy (SMA). "The label extension for Evrysdi to include pre-symptomatic ...
FDA Label Search - Food and Drug Administration The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA,... PDF label - Food and Drug Administration label Angioedema (e.g. swelling of the thro (800) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYRICA safely and effectively. See full... Carton and Container Labeling Resources | FDA - U.S. Food and Drug ... FDA's carton and container labeling specific resources on this webpage are primarily directed to industry staff who develop carton and container labeling for prescription drugs.* For other prescription drug labeling resources for industry such as those for the Prescribing Information, FDA-approved patient labeling, generic drug labeling ... FDALabel: Full-Text Search of Drug Product Labeling | FDA The following table lists the count of several common labeling types in FDALabel. * Includes Human OTC drugs approved for marketing through a New Drug Application (NDA), Abbreviated New Drug...
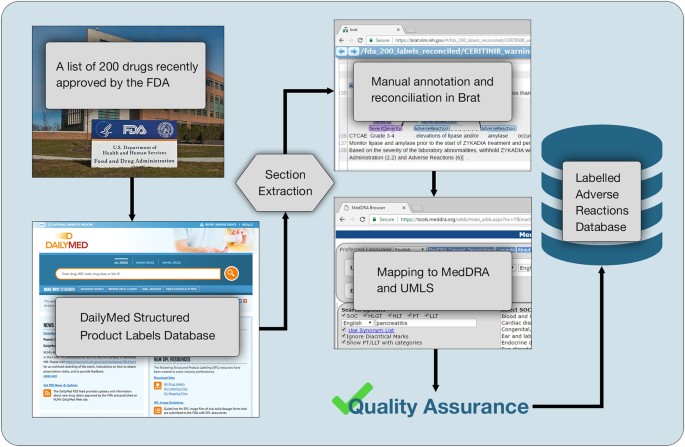
Drug Approval and Labeling | Cancer.Net Drug Approval and Labeling. Approved by the Cancer.Net Editorial Board, 07/2018. A primary role of the U.S Food and Drug Administration (FDA) is to make sure that all prescription and over-the-counter drugs in the United States are safe and effective. This includes drugs that are made up of chemical ingredients and biological products that are ... DailyMed DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary supplements, and medical foods. The NLM provides DailyMed to the public and does not accept advertisements. PDF (IVERMECTIN) - Food and Drug Administration sites where albendazole is approved for the treatment of strongyloidiasis of the gastrointestinal tract, and three controlled studies were carried out in the U.S. and internationally using thiabendazole as the comparative agent. Efficacy, as measured by cure rate, was defined as the absence of larvae in at least two follow-up stool DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... FDA's Prescription Drug Labeling Resources. This website provides over 100 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for human prescription drugs, including biological products. FDA's Drug Guidances. Guidance documents represent the FDA's current thinking on a ...
FDA Label Search-Ingredient Name - Food and Drug Administration 10903 New Hampshire Avenue. Silver Spring, MD 20993. Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government. For Press.
Types of FDA Drug Labeling and Their Requirements - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. [3] An example of FDA-approved labeling is the Professional Package Insert (PPI).
PDF Reference ID: 3397413 - Food and Drug Administration antipsychotic drugs are at an increased risk of death. SEROQUEL is not approved for elderly patients with dementia-related psychosis (5.1) Suicidal Thoughts and Behaviors • Increased risk of suicidal thoughts and behavior in children, adolescents and young adults taking antidepressants (5.2) •
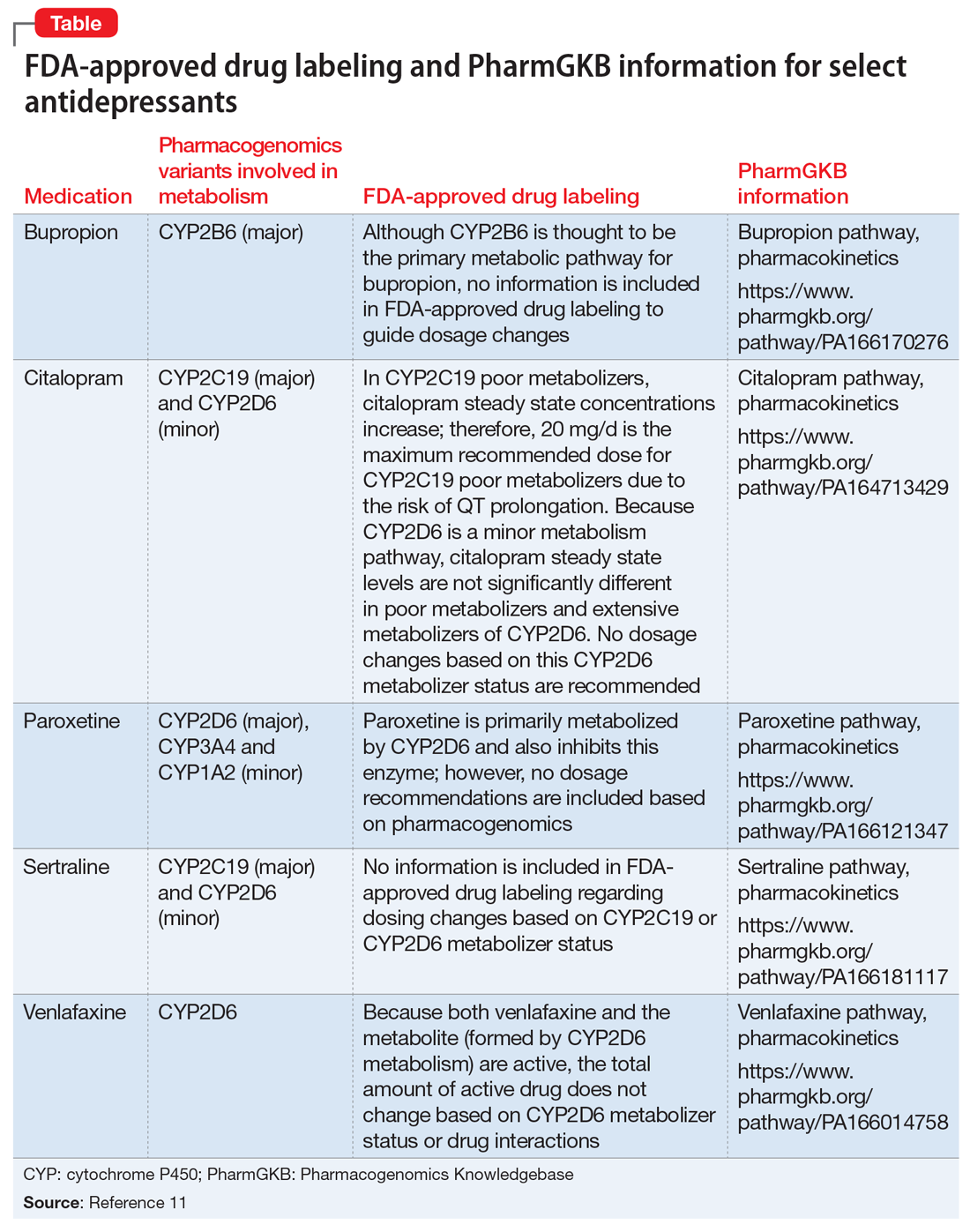
Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Pharmacogenomics (PGx) is a key subset of precision medicine that relates genomic variation to individual response to pharmacotherapy. We assessed longitudinal trends in US FDA approval of new drugs labeled with PGx information.
Drug Safety-related Labeling Changes (SrLC) Database Drug Safety-related Labeling Changes (SrLC) Database Overview: Updates to Safety Information in FDA-Approved Prescription Drug Labeling Contact FDA Toll Free (855) 543-3784, or (301) 796-3400...
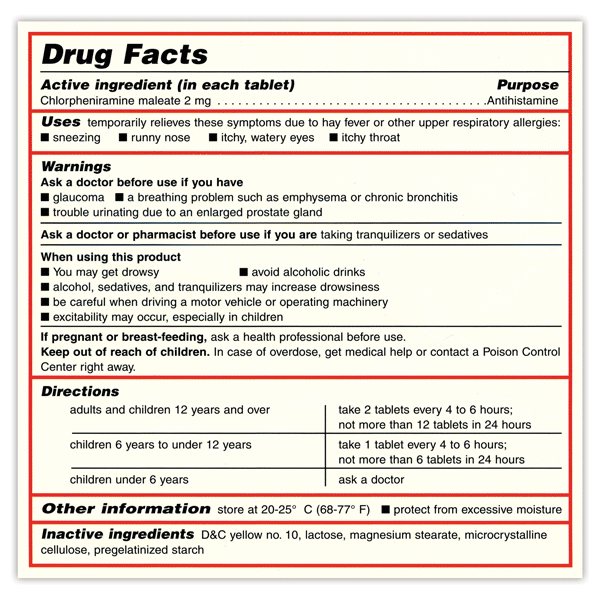
OTC Drug Facts Label | FDA In the Federal Register of March 1999, the Food and Drug Administration published the OTC Drug Facts Label regulation. This regulation required most OTC drug products to comply with the new format...
FDA Label Search-Package Code - Food and Drug Administration Vaccines, Blood & Biologics Animal & Veterinary Cosmetics Tobacco Products FDA Label Search FDA Home Search by NDC: (Type the 4 or 5 digit NDC Labeler Code with the hyphen (e.g., 0001-), the 8 or 9...
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
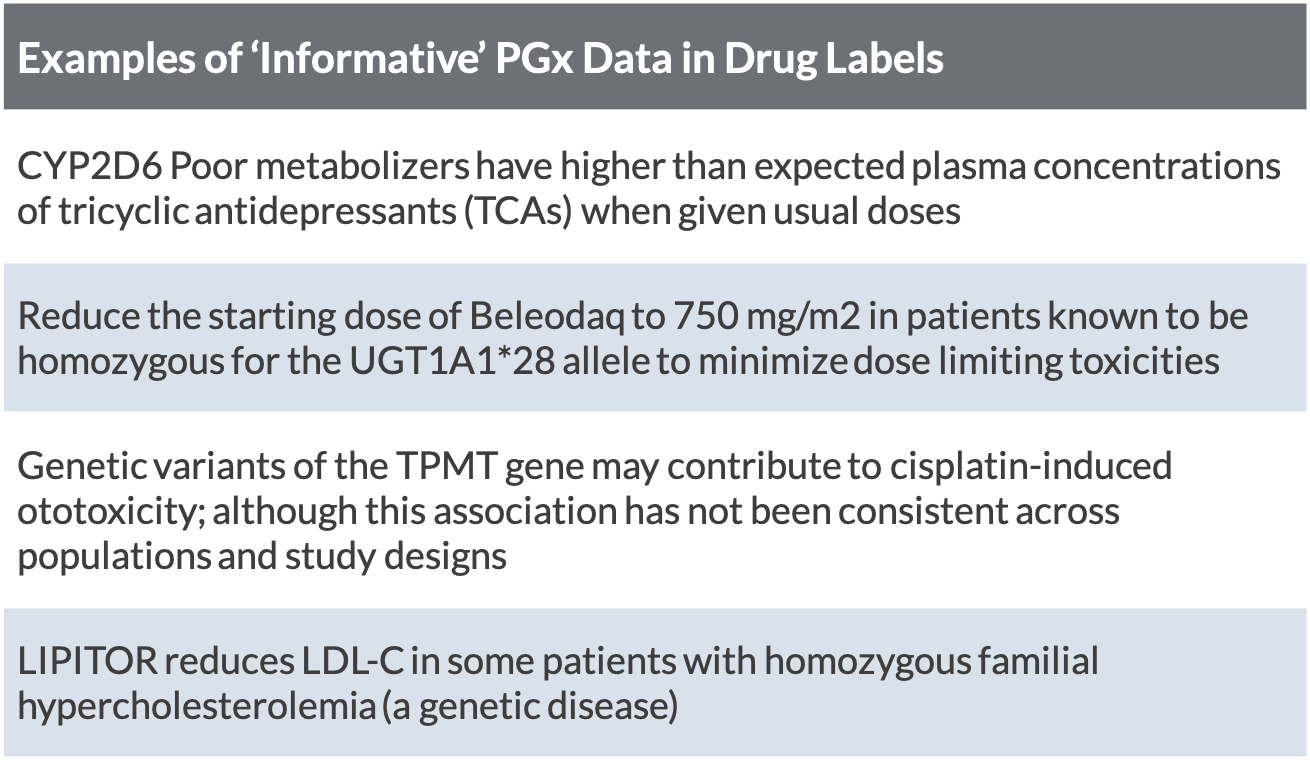
Pharmacogenetic Labeling of FDA-Approved Drugs - PMC The U.S. Food and Drug Administration recently marked 10 years since first updating the labeling for warfarin (often referred to as the "poster child" of pharmacogenomics) to include information regarding the potential impact of CYP2C9 and VKORC1 genetic variation on warfarin dosing requirements and risks.
PDF Neurontin (gabapentin) Capsules Neurontin (gabapentin) Tablets ... FDA Approved Labeling Text dated 03/01/2011 Page 3 . Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing.
FDA-approved drugs that interfere with laboratory tests: A ... - PubMed A total of 134 labels were positive, which indicated that the drug interferes with at least one clinical laboratory test. Antibacterial agents, psychotropic drugs and contrast media are the classes of drugs most likely to lead to DLTI. Urine was the clinical sample most frequently affected by DLTI. The FDA drug label is a source of information ...
What Information Should Be on Drug Labels? - MedicineNet Certain information must be included on a prescription drug label. The FDA requires prescription labeling to be printed with: Pharmacy information The doctor's information Instructions Physical description of the drug Federal caution statement Dates Pharmacy prescription number Number of pills Number of times the drug can be reordered
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions...
Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Drugs@FDA: FDA-Approved Drugs. Share; Tweet; Linkedin; Pin it; More sharing options. Linkedin; Pin it; Email; ... Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/18/2016: SUPPL-14: Manufacturing (CMC) Label is not available on this site. 04/22/2016:














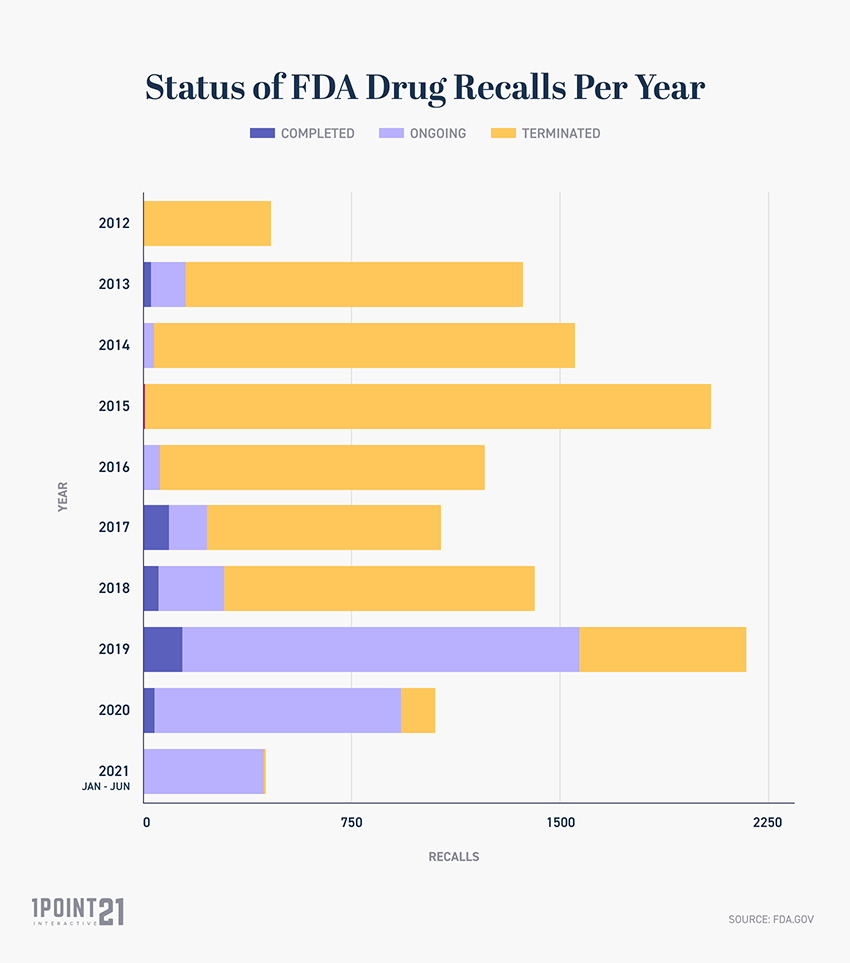
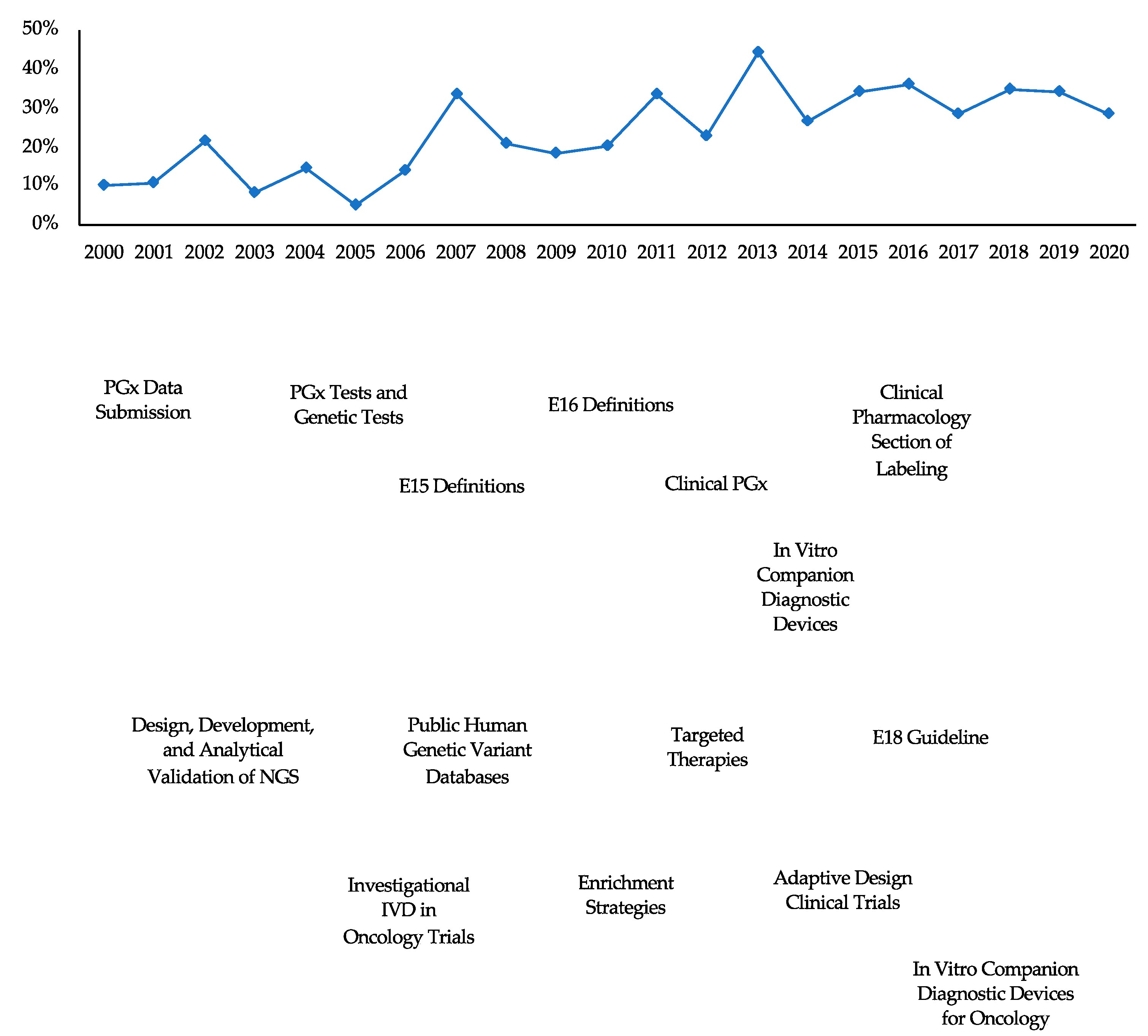









Post a Comment for "38 fda approved drug labels"